Tissue Engineering and Bone Repairing Scaffolds Made Possible with Reverse 3D Printing
When you have a bone fracture, the doctor will set the bone, insert a metal rod or plate, and use screws and pins to set it in place, as well as repair the damaged tissues. Depending on the severity of the fracture, the metal rod or plate will be permanent or removed later. After the surgery, the affected area will likely be covered in a cast.
There are many innovations in bone tissue repair methods, and doctors have more options today. One of the most common methods is bone grafting. In this method, surgeons place existing bone mass they gathered from another source and graft it to the section of bone that needs repair. But in most cases, it is difficult to collect enough bone needed for the operation.
Corals and marine sponges for bone repair
Researchers, using biomimicry developed a bone structure repairing method using marine sponges and corals as frames or scaffolds to support the regeneration of bone tissue. They are ideal for this type of bone repair because they are easy to integrate into the existing bone structures. Corals and marine sponges closely resemble human bones in terms of composition and porosity.
The surgeon shapes the sponge or coral into the shape of the area for repair. Other substances they need are stem cells or marrow stromal cells. The surgeon inserts the scaffold and the cells into the section of bone that was damaged. Over time, the bone tissue from the existing bone will expand into the scaffold. Otherwise, the stems cells will convert into bone cells on the scaffold depending on where they were implanted.
The marine sponge or coral will integrate into the bone structure as the bone heals, and will become part of the healthy bone.
Electrified scaffolding for bone healing
Some scientists developed a method of implanting materials using a scaffolding-like microstructure. Aside from giving bone cells a place to migrate into and grow, the scientist thought the method would work better with electrical stimulation.
But the earlier device included an integrated battery. Some have a hard-wired external source of power. However, with this method, the implants have to be surgically removed after the bone has healed.
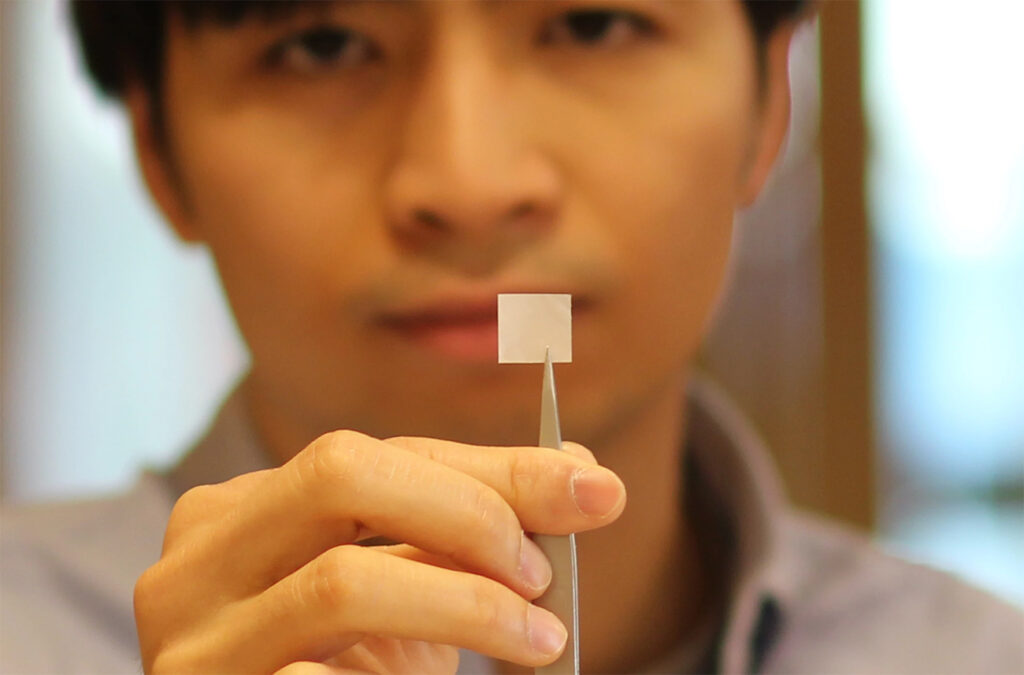
Scientists at the University of Connecticut, led by Thanh Nguyen, a biomedical engineer, created an electrified scaffold material that is wirelessly powered. The device does not need to be taken out because it is made with non-toxic, piezoelectric polymer nanofibers they call poly (L-lactic acid) (PLLA). The piezoelectric material can produce an electric charge when mechanical stress is applied.
The patient or the doctor can periodically use an external handheld device that will send ultrasound pulses. The vibrations generate a weak therapeutic electrical field that stimulates the bone cells to reproduce. Eventually, the implanted materials will dissolve, with natural bone replacing it.
The scientists are still testing the device using lab animals. They are now working on developing a scaffolding that will be more favorable to stimulating bone growth. The scientists are also looking into the possibility of using the technology to regrow other tissues, such as cartilage, nerves, and muscles.
Latest technology for repairing bone and tissues – 3D printing
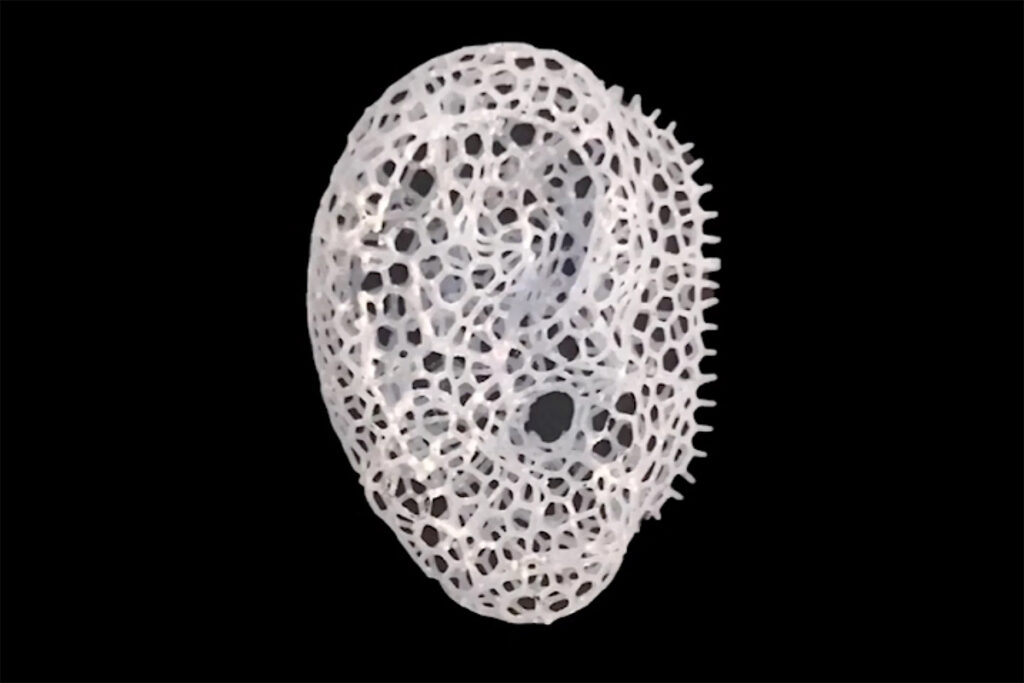
Another technology for bone and tissue repair a group of scientists is testing involves reverse 3D printing. With the technique they developed, they can create intricate implants for tissue engineering. They are still using scaffold-like implants but they have made the process faster with 3D printing.
The 3D printed implant is made of biodegradable, biocompatible materials with compounds that help cell growth. The internal structure of the implant is the same as that of natural bone, making it easier for the cells from adjacent bone tissue to migrate into the linked empty spaces and reproduce. As the natural cells continue to grow, the scaffolding material dissolves, leaving natural bone to fill the gap.
The walls of the empty spaces of the scaffold are intricate and thin, which makes it impossible for common 3D printers to create the intricate details. The scientists at the St. Vincent’s Hospital in Melbourne and Australia’s RMIT University developed the Negative Embodied Sacrificial Template 3D printing (NEST3D).
The process starts by 3D printing a negative template of the empty spaces of the scaffolding, using solid polyvinyl acetate (PVA) glue, which the nozzle of a basic 3D printer can handle. The high-school grade 3D printer has a bigger nozzle that allow the material to pass through easily. The high-end 3D printer has a small nozzle, making it difficult for the material to pass through.
The template is placed in a mold, and a biocompatible/biodegradable polymer liquid is poured into the mold to fill the gaps in the template. After the polymer solidifies, the PVA glue is washed off with water, dissolving the template, leaving the polymer skeleton with an intricate pattern.
Scientists are thinking the technology will someday be able to grow other replacement body parts in a laboratory. They are still in the testing stage but they look forward to making tissue engineering become a reality.