Glass as Hard as Diamond for the Hi-Tech Industry
Diamond is the hardest naturally occurring mineral on earth. It is made of pure carbon and has vital industrial applications because of its extreme hardness. Most diamonds come from Australia, Kinshasa (Democratic Republic of Congo), Russia, and South Africa. While highly-priced as a gemstone, several industries use diamond as well.
Among all-natural materials, diamond has the highest thermal conductivity. Likewise, diamond is chemically resistant. Most diamonds go to the jewelry industry, although there are diamonds that are used for industrial purposes. These are categorized as industrial diamonds generally used for cutting, drilling, polishing, and grinding other materials and substances.
These diamonds may be of gemstone quality, but their size and shape prevent them from being cut profitably. Instead, they are valued for their heat conductivity and hardness. Some are ground to micron-sized abrasive powders, and some are fashioned into cutting and drilling tools. For example, a diamond is a popular tool to cut glass.

A glass that is as hard as diamond
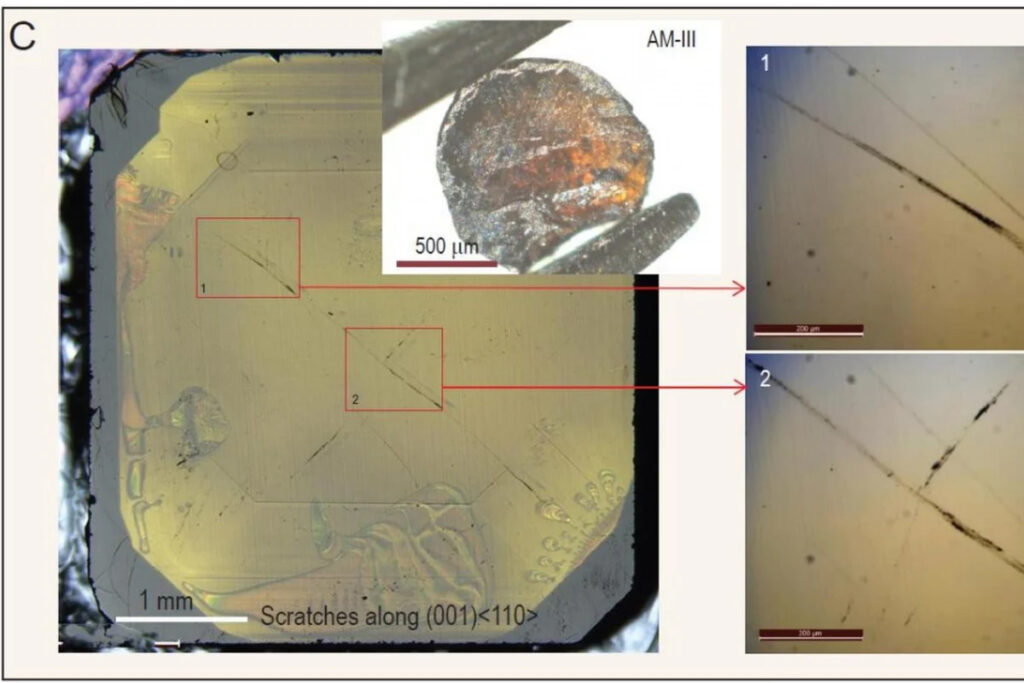
In a research laboratory in China, however, the reverse is happening. A team of international scientists from Russia, Germany, Sweden, and the United States collaborated with Chinese scientists at the Yanshan University in Qinhuangdao, Hebei province, to develop a glass material as hard as diamond. The glass they developed can easily leave a deep scratch on a diamond’s surface in their experiment.
The researchers believe that what they created is the hardest glassy material in the world. Made from pure carbon, they tentatively named the material AM-III. The material is a semiconductor with similar efficiency as silicon and can transfer electric current, which opens many possible applications in the hi-tech industry.
Properties of AM-III
AM-III is transparent and somewhat yellowish. In a Vickers hardness test, AM-III reached 113 gigapascals (GPa), whereas a natural diamond stone only got 50 to 70 GPa. Some artificial diamonds score 100 GPa.
As a material, glass is weak. Therefore, technically, AM-III is not glass. According to Professor Tian Yongjun of Yanshan University’s Center for High Pressure Science, AM-III is glass with crystals. He said that a diamond is a crystal with molecules and atoms that line up perfectly in one direction. On the other hand, glass has multiple directions and orders.
This internal structure of glass is similar to what AM-III has, so they call it glass. And this combination of order and disorder is what gives AM-III some unique traits.
AM-III’s unique properties set it apart from other strong artificial materials. In theory, graphene can withstand over 400 GPa. But its strength is only imminent when it is as thick as an atom. After that, it will turn into ordinary, soft graphite if several graphene layers are stacked together. This property limits the application of graphene as a thin coating over the surface of another rigid material.
In contrast, it is possible to produce AM-III in different sizes and shapes. Further, it is hard all around, unlike diamonds that have some weak surfaces.
Creating AM-III
Professor Tian said that they could produce several types of new materials using various combinations of the order-disorder structure. They achieved the highest strength in AM-III by giving it the highest proportion of molecules and atoms in order. However, they stopped increasing the orderly structure at a certain point because it would turn into a diamond and lose its semiconductivity and other properties.
The professor added that the process of making a super-strong glass is more complex than creating crystals. Using heat and pressure on cheap graphite will result in artificial diamonds. In the case of AM-III, they used fullerene, an allotrope. By the way, diamond, charcoal, and graphite are allotropes of carbon.
Fullerene has molecules that look like tiny and hollow soccer balls. The cage-like molecules are called “buckyballs” while the cylinder type is called “nanotube.” In its raw state, fullerene is soft and is used for some cosmetic products.
In creating AM-III, they used extreme heat and pressure to crush and blend fullerene. The application of heat and pressure took 12 hours to accomplish because they gradually increased the pressure and temperature until they reached the maximum. Then, it took another 12 hours to cool down the material. The result is the ultra-strong, ultra-hard semiconducting amorphous carbon material.
Professor Tian and his team are not new to this process. They created a boron nitride crystal in 2013 that was twice as hard as diamond. It remains the hardest material in the world that the naked eye can see.
The scientists envision various applications of AM-III, such as bulletproof windows, semiconductors, photoelectric devices, and other materials that need to perform under high temperatures and pressure.