A World’s First: Biodegradable Wireless Transient Pacemaker
George Washington and Northwestern universities researchers have recently published their research on developing the world’s first transient pacemaker. You can find the study in the journal of Nature Biotechnology. The device is battery-free, wireless, and fully implantable. The fantastic thing about the pacemaker is that it will disappear once it is no longer needed, absorbed by the body without any ill effects.
The pacemaker, which is made from thin, lightweight, and flexible material, could be implanted on patients who need temporary pacing while waiting for their permanent pacemaker or after their cardiac surgery. Every part of the pacemaker is biocompatible. The body’s biofluids will naturally absorb them within five to seven weeks. It does not have to be surgically removed.
A smart system
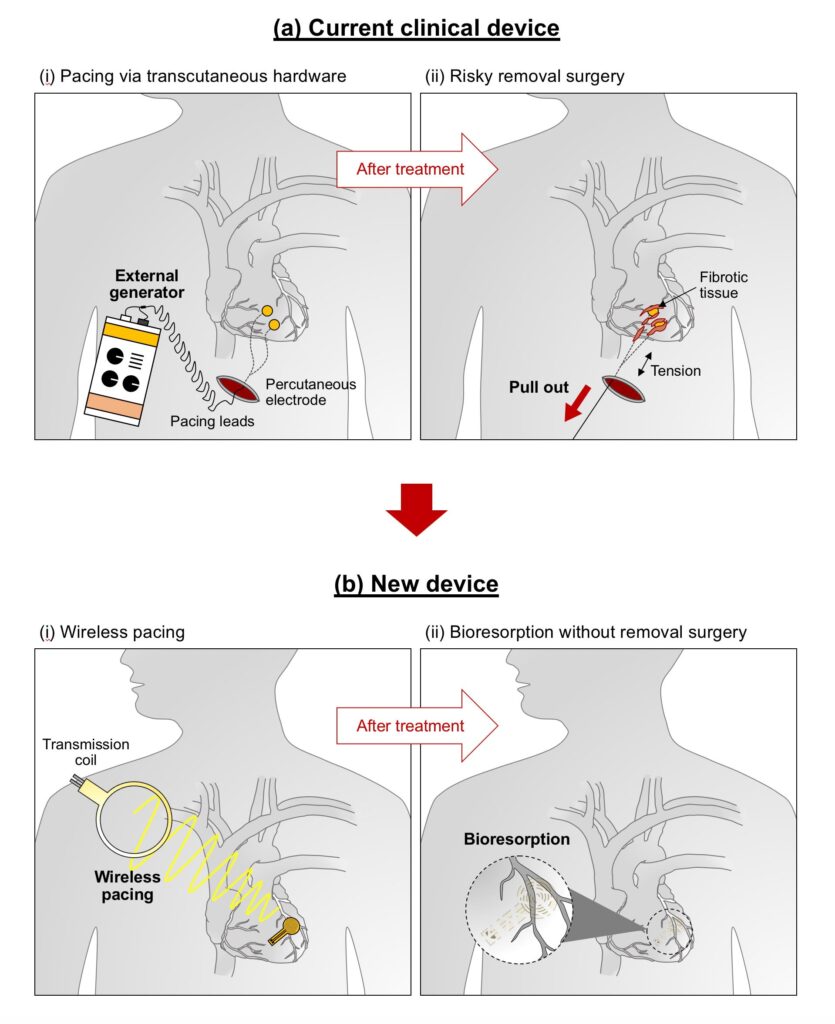
The device uses the same technology employed in RFID tags and electronic payments using smartphones. It wirelessly gets energy from an external, remote antenna using near-field communication protocols. The method removes the need for rigid hardware, wires (leads), and bulky batteries. These wires can introduce infections, and they can be covered with scar tissues, which can cause more damage during extraction.
Efficient device
The study demonstrates how efficient the pacemaker was when tested across small and large animal models. According to John A. Rogers, who led the development of the device, any hardware that is placed near or in the heart carries the risk of complications and infection. He said that the wireless device they developed removes the need for transdermal leads to aid in the surgical removal of the device, helping lower the surgery cost and improving the results of patient care.
Dr. Rishi Arora, a cardiologist and co-leader of the study, added that patients need temporary pacemakers after a drug overdose, a heart attack, or heart surgery. Once the patient stabilizes, the pacemaker is removed. The current practice is to insert a wire that will remain in place for three days to one week. With the method, there is a possibility that the wire will be dislodged or infected.
With the temporary pacemaker, they can open a new chapter in medicine and biomedical research. The biodegradable materials at the core of this technology make it possible for the researchers to a range of therapeutic and diagnostic transient devices to monitor the progression of therapies and diseases and delivering gene, cell therapies, electrical and pharmacological reprogramming.
Eliminating risks
After open-heart surgery, the patient needs temporary pacing. The current standard is for the surgeon to sew temporary pacemaker electrodes on the heart muscle while they are doing the surgery. The leads exit the front of the patient’s chest. The ends of the leads connect to an external pacing box, which delivers a current that controls the heart’s rhythm. The patient with a temporary pacemaker has to watch their activities and limit their movements to prevent dislodging the leads. When the patient no longer needs the temporary pacemaker, surgeons will remove the pacemaker’s electrodes.
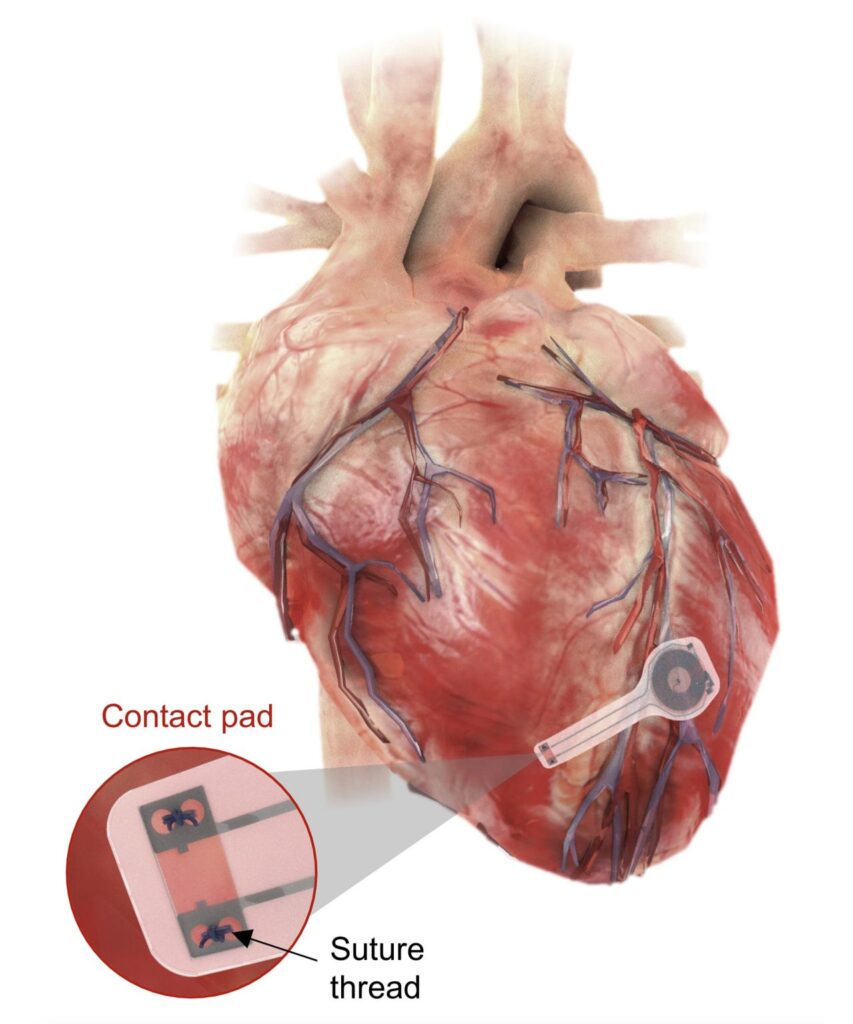
When using the temporary pacemaker developed by the George Washington and Northwestern universities, the health risks are eliminated. The temporary pacemaker is implantable. It is soft, flexible, thin, and light, weighing less than half a gram and only 250 microns thick. It attaches to the heart’s surface to deliver the needed electrical pulse, which they can activate remotely.
Welcome development
Cardiac surgeon Dr. Duc Thinh Pham from Northwestern Medicine welcomes the new device. He said that when it becomes available, it will make heart surgery patients very comfortable. They will be able to move freely, and their rehabilitation will be faster.
On the other hand, the researchers are looking at making changes to their invention to extend its application. Many stakeholders are hoping that the device will be successful and can be mass-produced soon.