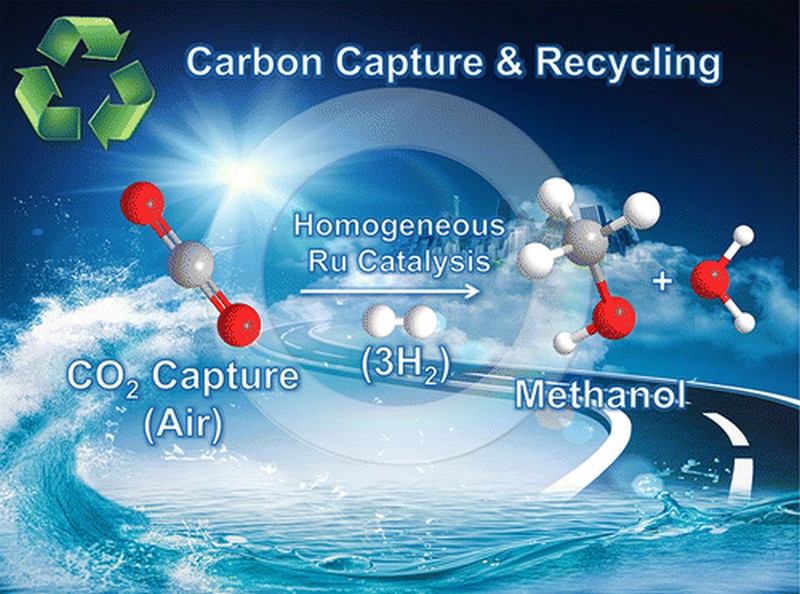
Carbon Dioxide in the Air Can Be Captured and Directly Converted into Fuel
A breakthrough has been achieved in carbon capture and conversion technology and is expected to help stimulate efforts in removing carbon dioxide, a greenhouse gas, from the atmosphere to slow down global warming. Researchers at the University of Southern California have demonstrated for the first time that it is possible and viable to capture CO₂ from the atmosphere and immediately convert it to fuel. Hitting two birds with one stone, the process helps reduce greenhouse gases while producing fuel that can be an alternative to gasoline.
One Step Towards a Methanol Economy
The study, led by University of Southern California chemistry professor G.K. Surya Prakash and Nobel laureate George Olah, is detailed in a paper published on the Journal of the American Chemical Society. It can be considered an important step in the possibility of making methanol the more dominant or at least a prominent energy source and raw material for various products.
Prakash takes pride in being part of the research project that has finally made it possible to directly capture CO₂ and convert it to CH3OH or methanol. “We have now done it!” Prakash proudly said in an interview with Phys.og. There have been similar efforts over the past decades to recycle carbon dioxide into useful products. However, one of the most attractive prospects has been the production of methanol by making carbon dioxide react with hydrogen gas. Methanol is an excellent product because of its applications in hydrogen storage and fuel cells as well as in serving as an alternative fuel.
*The image above is not the image of the paraphernalia used by the researchers. It is a direct methanol fuel cell.
At present, the global chemical industry outputs over 100 metric tons of methanol per year for various purposes. Because methanol is a simple compound, it serves as a building block for the production of larger compounds. Ethylene and propylene, which are used in the production of plastics and other products, are two of the most common large compounds that use methanol as a building block.
Hurdling the Challenges of Direct CO₂-to-CH3OH Process
To convert carbon dioxide to methanol, it is important to have a good homogeneous catalyst. This catalyst hastens the chemical reactions that lead to the production of methanol. The chemical reactions, however, generate high temperatures at around 150°C, which results in the decomposition of the catalyst used. This is where the researchers spent most of their time and effort.
To counter the problem of catalyst decomposition, they developed a stable catalyst that is based on ruthenium. Being a polyvalent metallic element, ruthenium is able to withstand high temperatures. It also exhibits good stability that’s why it can be used repeatedly to sustain the continuous production of methanol.
The other catalysts tried by the researchers only managed to produce formic acid or CH₂O₂. With the ruthenium-based catalyst developed by the researchers, they were able to convert carbon dioxide to methanol without having to repeatedly reintroduce the catalyst to compensate for the decomposition. Even better, they were also able to convert carbamates or alkylammonium bicarbonates directly into methanol.
More Studies Needed
Of course, this new breakthrough is not going to become implementable in a ready-to-use technology soon. More studies are needed to make the technology ready for actual use. The researchers are still planning to reduce the catalyst operating temperature and make the process more efficient. They intend to bring the catalyst operating temperature down to the 100°C to 120°C range. They also plan to develop a system that is “preparatively useful,” wherein the solvents or reagents used are not lost.
Ultimately, to make this breakthrough into a useful technology, the goal is to develop an “anthropogenic carbon cycle” that will supplement the natural carbon cycle to help lower the global-warming-inducing carbon dioxide present in the atmosphere. For the uninitiated, carbon on earth is perpetually recycled naturally, i.e. it is being continuously exchanged, reused, and recycled among oceans, animals and other living organisms, and the atmosphere. However, humans tend to use carbon in its different forms at a very fast rate that natural carbon cycle alone is incapable of keeping the balance. That’s why the breakthrough achieved by the University of Southern California researchers spells big progress in the endeavor to address global warming while finding alternative energy sources.
Once this breakthrough is made into a technology that is accessible to governments, businesses, or even individuals, more will be taking an active part in removing carbon from the air since the process will yield something of value (methanol). Hopefully, it materializes into something that can truly create a significant impact in addressing the problem of excessive greenhouse gases in the atmosphere.